electron configuration of iron|Electron Configuration Chart of All Elements (Full Chart) : Tuguegarao In order to write the Mg electron configuration we first need to know the .
Download Hidden Assets S02E05 1080p RTE -DL x264-W4N70Ks or any other file from TV category. HTTP download also available at fast speeds. . 1 month ago Hidden Assets S02 720p Rip x264-GalaxyTV. . 7 months ago Hidden Assets S01 1080p H264-Zero00. size 3.29 GiB in TV TV Packs. Show Comments. No comments .
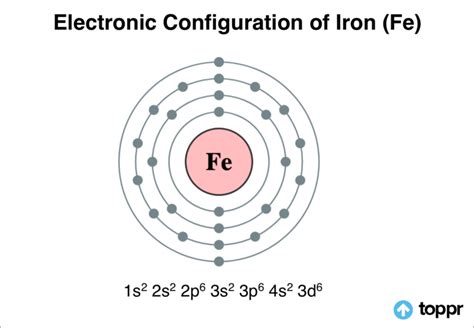
electron configuration of iron,Learn how to write the electron configuration for iron and its ions using the period table or an electron configuration chart. See the video, examples and step-by-step tutorial for writing the electron configurations.In order to write the Calcium electron configuration we first need to know the .Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s .How to Write the Electron Configuration for Nitrogen (N) Nitrogen is the seventh .When we write the configuration we'll put all 19 electrons in orbitals around the .In order to write the Mg electron configuration we first need to know the .Chlorine (Cl) - Electron Configuration for Iron (Fe, Fe2+, and Fe3+) - UMDLearn the electronic configuration of iron (Fe) with 26 electrons, its oxidation states, allotropic forms and applications. Find out how iron forms bonds, . Mar 23, 2023 Learn how to write the electron configuration of iron (Fe) using the Bohr model of orbitals and the Aufbau principle of orbitals. Compare the differences and similariti.
electron configuration of iron Electron Configuration Chart of All Elements (Full Chart)Learn how to write the electronic configuration of iron and its oxidation states, valency, and applications. Find out the reduced form of iron and the difference between iron and .
Learn how to write the electron configuration of iron using the noble gas shortcut and the energy levels of the orbitals. See the answer, the long version, and the corrected diagram. The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. So, .
The Electronic Configuration of Iron. A chemical element with an atomic number 26 and a symbol Fe represents it, Iron is a very common element that is found. .

Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic structure. The chemical symbol for Iron is Fe. .By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state electron configuration and orbital diagram . In order to write the electron configuration for Iron (Fe) we first need to know the number of electrons for the Fe atom (there are 26 electrons). When we.electron configuration of ironThe first three quantum numbers of an electron are n=1, l=0, m l =0. Only two electrons can correspond to these, which would be either m s = -1/2 or m s = +1/2. As we already know from our studies of quantum numbers .Electron Configuration Chart of All Elements (Full Chart)The first three quantum numbers of an electron are n=1, l=0, m l =0. Only two electrons can correspond to these, which would be either m s = -1/2 or m s = +1/2. As we already know from our studies of quantum numbers . The iron electron configuration is one of the other significant areas of study for scholars of chemistry. The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. So, the Iron electron configuration is basically the 1s2 2s2 2p6 3s2 3p6 3d6 . Electronic Iron configuration will be : \[1s^{2} 2s^{2} 2p^{6} 3s^{2} 3p^{6} 4s^{2} 3d^{6}\]. Iron, a chemical substance or element, is a metal generally represented by its symbol Fe. Its symbol is short for Ferrum which is a Latin term. The atomic number of iron is 26. Iron is a member of group 8 and the first transition series of the periodic . Filling Transition Metal Orbitals. The electron configuration for the first row transition metals consists of 4s and 3d subshells with an argon (noble gas) core. This only applies to the first row transition metals, adjustments will be necessary when writing the electron configuration for the other rows of transition metals. The noble gas before the .
Figure \(\PageIndex{7}\): A core-abbreviated electron configuration (right) replaces the core electrons with the noble gas symbol whose configuration matches the core electron configuration of the other element. . Iron(II) loses two electrons and, since it is a transition metal, they are removed from the 4s orbital Fe 2+: .Protons and Neutrons in Iron. Iron is a chemical element with atomic number 26 which means there are 26 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.Aufbau Principle. Aufbau comes from the German "structure" or building up and Aufbauprinzip, is the "building up principle" where we assign electrons to the lowest energy orbitals first, and then successively add them to higher energy orbitals until we run out of electrons.This way the electron configuration is that of the lowest energy and so the .Its 26 electrons are arranged in the configuration [Ar]3d 6 4s 2, of which the 3d and 4s electrons are relatively close in energy, and thus a number of electrons can be ionized. [16] Iron forms compounds mainly in the oxidation states +2 (iron(II), "ferrous") and +3 (iron(III), "ferric").

The electron configurations and orbital diagrams of these four elements are: The alkali metal sodium (atomic number 11) has one more electron than the neon atom. . Iron(II) loses two electrons and, since it is a transition metal, they are removed from the 4s orbital Fe 2+: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 = 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6.
Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. [1] For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .
For example, the electron configuration of iron is Fe is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. The total number of valence electrons for iron is 8: 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the (n-1) d orbital, that is, 3d. Using abbreviated electron configuration (or noble gas configuration) to identify valence . The ground state electron configuration of Fe is: "1s"^2"2s"^2"2p"^6"3s"^2"3p"^6"3d"^6"4s"^2" For all but about 20 transition metals, the Aufbau diagram is a useful tool that helps to determine the .
Consider the electronic structure of neutral iron and iron (III). To write the electronic structure for Fe 3 +: Fe: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2; . W. H. Eugen Schwarz: The Full Story of the Electron Configurations of the Transition Elements: Journal of Chemical Education, Vol. 87 No. 4 April 2010. Write the electronic configurations of: neutral iron, iron(II) ion, and; iron(III) ion. Answer. The atomic number of iron is 26 so there are 26 protons in the species. Fe: [Ar] 4s 2 3d 6; . Electron configurations of unpaired electrons are said to be paramagnetic and respond to the proximity of magnets.Electronic configuration of the Iron atom in ascending order of the levels: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2 Reduced electronic configuration Fe: [Ar] 3d 6 4s 2. Below is the electronic diagram of the Iron atom Distribution of electrons over energy levels in the Fe atom 1-st level (K): 2Noble Gas Configuration. The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (Table \(\PageIndex{1}\)). The first ten electrons of the sodium atom are the inner-shell electrons and the configuration of just those ten electrons is exactly the same as the configuration of the element neon \(\left( Z=10 \right)\). Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.
electron configuration of iron|Electron Configuration Chart of All Elements (Full Chart)
PH0 · What is the electron configuration of iron?
PH1 · Iron – Electron Configuration and Oxidation States – Fe
PH2 · Iron Electron Configuration (Fe) with Orbital Diagram
PH3 · Electronic Configuration of Iron: Fe element
PH4 · Electronic Configuration of Iron
PH5 · Electron Configuration for Iron (Fe, Fe2+, and Fe3+)
PH6 · Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions)
PH7 · Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions)
PH8 · Electron Configuration Chart of All Elements (Full Chart)
PH9 · 3.1: Electron Configurations